A recent review article in Nature Neuroscience, revealed that stress on the endoplasmic reticulum (ER), caused by an incorrect DNA sequence in myelinating nerve cells, is a key factor in the development of various neural demyelinating disorders such as Pelizaeus-Merzbacher Disease and Multiple Sclerosis.
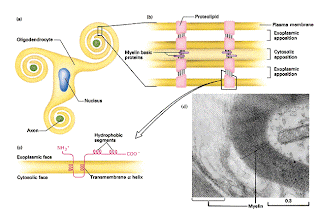 Myelin is a unique lipid sheath that wraps around the axons of neurons in the Central Nervous system (CNS) and Peripheral Nervous System (PNS). They are essential for the fast conduction of electrical impulses (action potential) as well as the maintenance of the axonal integrity. In the CNS, the proteolipid protein (PLP) plays a major factor in the compaction and wrapping of the myelin secreted by brain cells called oligodendrocytes. Mutations in the PLP gene (PLP1) has been shown to synthesise non-functional proteins which are unable to be folded correctly within the oligodendrocyte’s ER. These mutant proteins then accumulate in the ER, disturbing the cell’s endoplasmic reticulum ultrastructure and place increased pressure on the ER. The cell responds to this with the ‘unfolded protein response’ (UPR) either in an adaptive or apoptosis (suicidal) pathway.
Myelin is a unique lipid sheath that wraps around the axons of neurons in the Central Nervous system (CNS) and Peripheral Nervous System (PNS). They are essential for the fast conduction of electrical impulses (action potential) as well as the maintenance of the axonal integrity. In the CNS, the proteolipid protein (PLP) plays a major factor in the compaction and wrapping of the myelin secreted by brain cells called oligodendrocytes. Mutations in the PLP gene (PLP1) has been shown to synthesise non-functional proteins which are unable to be folded correctly within the oligodendrocyte’s ER. These mutant proteins then accumulate in the ER, disturbing the cell’s endoplasmic reticulum ultrastructure and place increased pressure on the ER. The cell responds to this with the ‘unfolded protein response’ (UPR) either in an adaptive or apoptosis (suicidal) pathway.If the adaptive pathway is undertaken, the cell uses methods such as expanding the ER or transcribing cytoprotective genes to try and cope. Despite these measures, the myelin sheath will be structurally defective; and whilst the cell may live, its messaging transmission capabilities would be hindered. If the UPR deems the cells as ‘too-stressed-out’ it will initiate cell apoptosis. While the exact methods on how the UPR chooses whether to initiate apoptosis or adaption is unclear, it is generally postulated that the outcome of ER stress is related to the developmental status of the cell.
From the catching of a ball and speech, to the typing at a keyboard, all these are made possible by the firing of neurons and their protective myelin sheaths. Without the myelin, the electrical impulses are chopped and slow resulting in motor and neural difficulties. Without PLP, myelin would not be able to be compacted and wrapped correctly and excess junk PLP can cause cell death from ER stress. Furthermore, as this misfolding has been shown to be a genetic mutation in PLP1, and this has implications in the heritability of neurodegenerative disorders but it also opens some avenues for the testing of these diseases. Though considerable progress has been made in the understanding of the pathways a cell undertakes in relation to ER stress, recent studies have begun to suggest that the manipulation of these signalling pathways has a potential to be therapeutic.
...and so as an exciting sidenote, groundbreaking research into the PLP 1 gene sequence and its role in relation to the neurodegenerative disease, Multiple Sclerosis (MS) is actually currently being undertaken here, in Queensland, at the University of Queensland’s Centre for Clinical Research (UQCCR)by Dr Judith Greer’s Group. They hope to link the PLP1 gene mutation with MS and possibly also develop a drug to reduce MS's relapse attack severity. Though largely untouched by the Australian research community, the PLP gene and protein will most certainly play a key role in the research of certain neurodegenerative diseases, such as MS, now and into the future.
Article: Lin, W., & Popko, B. (2009). Endoplasmic reticulum stress in disorders of myelinating cells. Nature Neuro. , 379-383.
(http://www.nature.com/neuro/journal/v12/n4/abs/nn.2273.html) [Abstract only]
University of Queensland’s Centre for Clinical Research
http://www.uqccr.uq.edu.au/
Dr Judith Greer’s Group
http://www.uqccr.uq.edu.au/about-uqccr/staff/staff-profiles/dr-judith-greer.aspx#description
By Antony Ji (42004192)